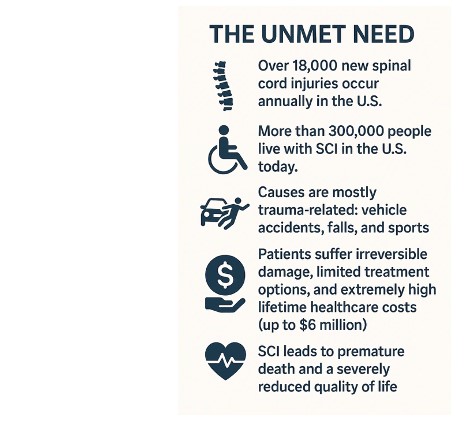
Bringing hope to spinal cord injury (SCI) patients worldwide
Matricelf’s autologous regenerative platform offers a transformative approach—using each patient’s own cells and tissues to engineer functional neural implants. Our goal: to restore independence, reduce the economic burden, and redefine the future for SCI patients worldwide.
Engineering 100% autologous neural implants
The process begins with a small biopsy, taken from the patient’s omentum tissue by an abdominal incision. The omental biopsy undergoes a proprietary decellularization process that results in thermo-responsive hydrogel formation, while induced pluripotent stem cells (iPSCs) are re-programmed from patient’s mature cells.
Ex-vivo differentiation of iPSCs within the thermo-responsive hydrogel leads to the generation of a functional, patient-specific neural implants that are capable of bridging injured spinal tissue.